Clinical bottom line
Children in the community with signs of mildly infected eczema respond well to topical emollient and corticosteroids. Adding oral or topical antibiotics appears to have little clinical benefit.
Clinical scenario
As a nurse, you notice some children’s symptoms improve with just the usual topical emollients and corticosteroids. You decide to review the evidence on the effectiveness of antibiotics for treating mildly infected eczema.
Question
In children with signs of infected eczema, do antibiotics, in addition to topical emollient and corticosteroids, improve symptoms?
Search strategy
PubMed Clinical Queries (therapy, narrow): eczema AND antibiotics
Citation
Francis NA et al. (2017). Oral and Topical Antibiotics for Clinically Infected Eczema in Children: A Pragmatic Randomized Controlled Trial in Ambulatory Care. Ann Fam Med, 15(2), 124-130. doi: 10.1370/afm.2038
Study summary
The CREAM (Children with Eczema Antibiotic Management) study was a three-arm, double-dummy, blinded, randomised controlled UK trial involving children with atopic eczema presenting with clinically suspected infected eczema. Included were children (mean age was 3.1 years) with signs of infected eczema that could include: failing to respond to standard treatment with emollients and/or mild-to-moderate topical corticosteroids; a flare in the severity or extent of the eczema; weeping or crusting. Excluded were those who had recently used potent topical corticosteroids or antibiotics, had features of severe infection, or significant comorbidities. Of 171 children assessed, 113 were randomised to one of three study arms.
Standard care: Topical corticosteroid: hydrocortisone one per cent for the face and clobetasone butyrate 0.05 per cent (or equivalent) for other parts of the body, and an emollient of parents’ choice (non-antimicrobial). Follow-up at two weeks, four weeks by research nurse and at three months via clinical record review.
Intervention 1: (n=36) Oral antibiotic and topical placebo (oral antibiotic group): flucloxacillin (floxacillin) suspension (250mg/5mL), or erythromycin suspension (250mg/5mL) for those with penicillin allergy, for seven days using age-adjusted doses.
Intervention 2: (n =37) Topical antibiotic and oral placebo (topical antibiotic group): two per cent fusidic acid cream, applied three times a day for seven days.
Control: (n= 40) Oral and topical placebos (control group): Placebos were matched by taste and appearance.
Primary outcomes: Eczema severity (skin redness, cracking, soreness, itch, sleep disturbance, oozing or weeping, bleeding, and fever) at two weeks measured by the patient-oriented eczema measure (POEM).
Secondary outcomes: Eczema Area and Severity Index (EASI), Infants Dermatitis Quality of Life instrument or Children’s Dermatology Life Quality Index (depending on age of child); Dermatitis Family Impact (DFI)18 instrument, and the Atopic Dermatitis Quality of Life instrument; adverse events.
Study validity
Randomisation – yes; allocation concealment – yes (information from protocol); complete follow-up – small loss to follow-up for primary outcome; intention-to-treat analysis – all participants with baseline and two-week POEM scores were included; blinding – yes, children, parents, outcome assessors; equal treatment between groups – appears so; groups similar at baseline – yes, including POEM scores, infection features, and skin swab S. Aureus results. Overall, a high-quality study.
Results
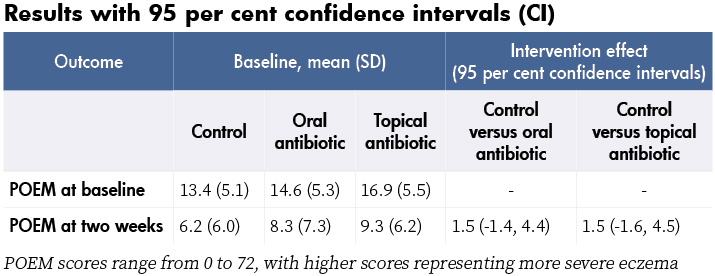
At baseline, 104 children (93 per cent) had one or more of the following: weeping, crusting, pustules, or painful skin. Also 70 per cent had S. aureus isolated from a skin swab and 27 per cent of those were resistant to fusidic acid. At two weeks, POEM scores had reduced (improved) in all three groups, with no significant differences in the POEM scores of the two intervention groups compared with control (see table). No significant differences in POEM scores were found at four weeks and three months either, or in secondary outcomes or adverse events. No serious adverse events were reported.
Comments
- Children recovered quickly, regardless of treatment group.
- Topical corticosteroids use was measured and similar in each group. Adherence to oral and topical antibiotic (or matched placebos) was 61.3 per cent and 81.8 per cent respectively. Adjusting for adherence differences did not change the results.
- Although recruitment targets were not met, the lower boundaries of the confidence intervals (-1.4 and -1.6) are less than an effect from treatment considered to be important to patients (published minimal clinically important difference for POEM is three), suggesting that a larger study would not change these results.
- Results do not apply to children with severe infection.
Reviewer: Cynthia Wensley RN, MHSc. Honorary Professional Teaching Fellow, University of Auckland and PhD Candidate, Deakin University, Melbourne. [email protected]